A Simple Technique to Minimize Side Effects in Prostate Cancer Treatment: The SABRE Trial

By: David Grew MD MPH
“[The hydrogel spacer] is injected between the prostate and rectum, creating a small space that helps shield the rectum from radiation exposure."
∗ ∗ ∗
As a radiation oncologist, one of the most important aspects of my job is not only to treat cancer but to ensure that patients experience as few side effects as possible from the treatment itself. One of the great innovations in prostate cancer treatment in my ten years in clinical practice is the widespread availability of stereotactic body radiation therapy (SBRT). With this, patients can receive curative treatment in just 5 treatments, instead of over many weeks (in some cases up to 9 weeks!).
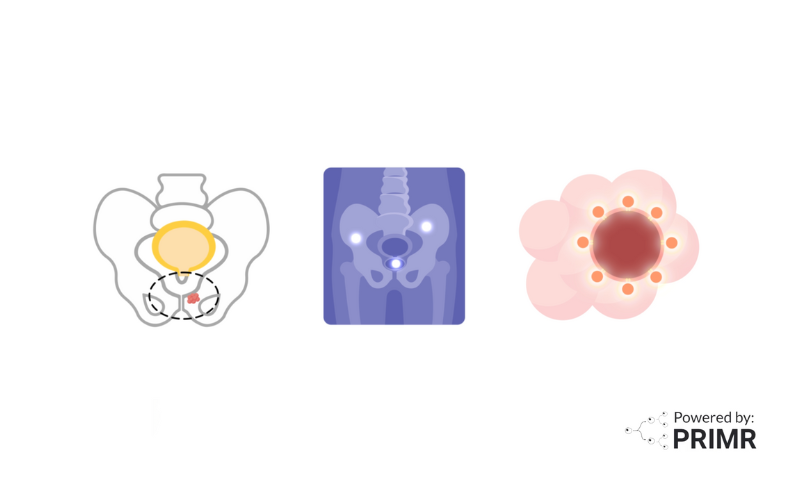
SBRT delivers a very high dose of radiation to the prostate, while shaping the beam around surrounding normal organs, like the rectum and bladder. Historically, a small minority of patients (somewhere around 2 percent) experienced radiation injury to the rectum. While rare, radiation proctitis can be incredibly uncomfortable and lead to terrible quality of life, even in spite of prostate cancer cure.
Fortunately, clinical trials such as the SABRE trial are exploring new ways to reduce these side effects and improve the quality of life for prostate cancer patients. The SABRE trial is investigating the use of an iodinated hydrogel spacer during stereotactic body radiation therapy (SBRT) to see if it can minimize the impact of treatment.
Understanding the SABRE Trial
The SABRE trial is an ongoing clinical study designed for men with intermediate-risk prostate cancer who are receiving SBRT. SBRT is a highly targeted form of radiation therapy that delivers precise and intense doses of radiation to destroy cancer cells while minimizing exposure to nearby healthy tissues. Although SBRT is both safe and effective, it can cause some side effects, including rectal pain, diarrhea, urinary urgency, and even erectile dysfunction, which can negatively impact a patient's quality of life.
A Potential Solution to Minimize Side Effects
One of the key questions in the SABRE trial is whether the use of an iodinated hydrogel spacer can reduce these side effects. This hydrogel spacer, already approved for use during conventional external beam prostate cancer radiotherapy, is a temporary implant made from a soft, water-based gel. It is injected between the prostate and rectum, creating a small space that helps shield the rectum from radiation exposure.
The procedure to place the hydrogel spacer is minimally invasive, involving the use of a thin needle guided by an ultrasound probe. This temporary spacer stays in place for about six months, after which the body naturally absorbs it. The hope is that this added buffer will lower the chances of rectal damage and other complications from SBRT, allowing patients to complete their treatment with fewer side effects.
Why the SABRE Trial Matters
The primary goal of the SABRE trial is to improve the quality of life for prostate cancer patients undergoing radiation therapy. If the iodinated hydrogel spacer proves to be effective, it could lead to widespread adoption of this technique as part of SBRT, providing patients with improved quality of life as they enjoy the long horizon of prostate cancer cure.
For patients facing prostate cancer, minimizing the side effects of treatment can be just as important as tackling the disease itself. The SABRE trial holds promise in addressing this concern by investigating a new way to protect healthy tissue during radiation therapy. If successful, this trial could pave the way for future treatments that offer better outcomes with fewer side effects.
To learn more about the SABRE trial and its impact on prostate cancer treatment, watch the video we made here.
Hire PRIMR to create custom video content for your clinical trial or medical product today.
FAQs:
How does the hydrogel spacer compare to other methods used to reduce side effects in radiation therapy for prostate cancer?
The hydrogel spacer, specifically designed to create a physical barrier between the prostate and rectum, is one of the most advanced methods for reducing rectal damage during prostate cancer radiation therapy. Other methods include the use of rectal balloons, positioning techniques, or lower radiation doses, which may reduce exposure but can sometimes compromise the effectiveness of treatment. The hydrogel spacer is minimally invasive, is absorbed by the body over time, and has been shown in other studies to significantly reduce the likelihood of rectal complications compared to these older methods.
Are there any risks or side effects associated with using the hydrogel spacer itself?
While the hydrogel spacer is generally considered safe and well-tolerated, there are some potential risks, although they are relatively rare. These can include infection, bleeding, or discomfort at the injection site, as well as potential reactions to the hydrogel material. However, the procedure to insert the spacer is minimally invasive, and the spacer is designed to be absorbed by the body over time, reducing the risk of long-term complications. The majority of patients experience few if any issues related to the spacer itself.
How widely available is the hydrogel spacer, and is it covered by insurance?
The hydrogel spacer has been approved by the FDA and is already being used in clinical practice, particularly for patients undergoing radiation therapy for prostate cancer. Its availability may vary depending on the treatment center and region. In terms of insurance coverage, most major health insurance plans, including Medicare, now cover the cost of the hydrogel spacer for prostate cancer patients as part of their radiation therapy. Patients are encouraged to check with their insurance provider to confirm coverage and potential out-of-pocket costs.
Other Posts
Nuclear Medicine: PSMA Treatment Explained from a Doctor’s Perspective
Nuclear Medicine: PSMA Imaging and Its Impact on Prostate Cancer Care