The CLARIFY Trial: Advancing Imaging for High-Risk Prostate Cancer

By: David Grew MD MPH
"This improved accuracy helps doctors avoid unnecessary treatments and tailor therapy to each patient’s specific needs."
∗ ∗ ∗
As a radiation oncologist, I frequently discuss treatment options with patients diagnosed with high-risk prostate cancer. One of the most challenging aspects of managing this disease is determining the true extent of cancer spread. I remember a patient asking, “Doc, how can we be sure the cancer hasn’t already spread before we plan surgery?” This is a critical question because undetected cancer spread can lead to suboptimal treatment decisions.
The CLARIFY trial is investigating a new imaging agent, 64Cu-SAR-bisPSMA, designed to improve prostate cancer detection. This study has the potential to refine how we identify cancer spread and ultimately improve treatment strategies. If successful, it could lead to better surgical planning and more personalized treatment approaches.
The Challenge with Current Imaging
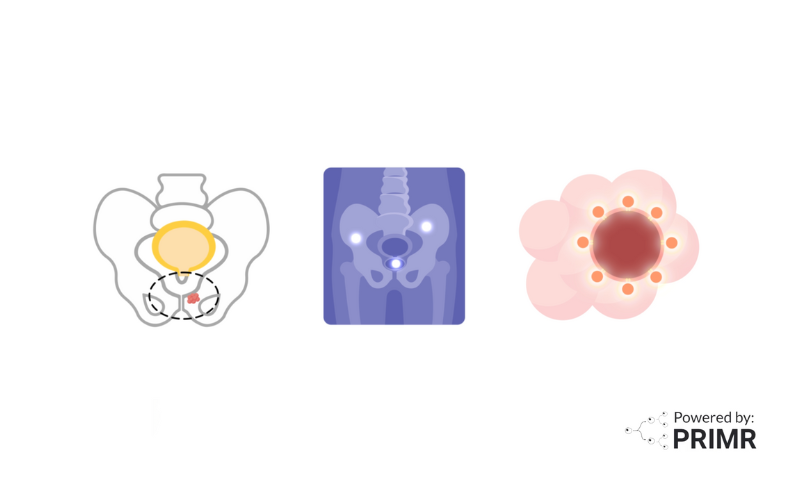
When someone is diagnosed with prostate cancer, doctors use a combination of PSA levels, Gleason scores, and imaging tests like CT or MRI scans to assess whether the cancer has spread. For patients with high or very high-risk prostate cancer, there’s a greater chance that the disease has moved beyond the prostate to the lymph nodes or other parts of the body.
While standard imaging tests are helpful, they don’t always detect small or early metastases. This can leave doctors with an incomplete picture of the disease, making it harder to choose the most effective treatment plan.
The Role of PSMA PET Scans
A more advanced way to check for prostate cancer spread is through PSMA PET scans. These scans use a special tracer that attaches to prostate cancer cells, lighting them up on the scan so doctors can see where the cancer has spread. Compared to traditional CT or bone scans, PSMA PET scans offer much sharper images, making it easier to detect cancer in the lymph nodes, bones, or distant organs. This improved accuracy helps doctors avoid unnecessary treatments and tailor therapy to each patient’s specific needs.
That said, current PSMA tracers aren’t perfect. They might not always pick up tiny tumors or microscopic cancer deposits, which means some early metastases could go undetected. Plus, their imaging window is relatively short, which can make scheduling tricky. This is where the CLARIFY trial’s new imaging agent, 64Cu-SAR-bisPSMA, could make a difference.
Introducing 64Cu-SAR-bisPSMA and the CLARIFY Trial
The CLARIFY trial is testing 64Cu-SAR-bisPSMA, a next-generation PSMA tracer that could provide clearer and more reliable images than current options. Some of its potential benefits include:
A longer imaging window. It stays in the body longer, allowing scans at both 1 hour and 24 hours post-injection.
Better sensitivity. It may improve the ability to detect small metastases, leading to more accurate staging.
More informed treatment decisions. If doctors can see the full extent of the cancer more clearly, they can plan surgery or other treatments with greater precision.
Patients in the CLARIFY trial receive an injection of 64Cu-SAR-bisPSMA and undergo PET/CT scans at two time points: one soon after injection and another the next day. This allows doctors to see if the extra time provides a clearer view of cancer spread. These results are then compared to standard imaging methods and, for some patients, checked against surgical findings to confirm accuracy. The study also includes follow-ups to ensure patient safety and track any side effects. By the end of the trial, researchers hope to determine if 64Cu-SAR-bisPSMA can set a new standard for prostate cancer imaging.
What This Means for Future Patients
If 64Cu-SAR-bisPSMA proves to be more effective, it could significantly improve the way prostate cancer is diagnosed and treated. More precise imaging would help doctors confidently decide who truly needs surgery or radiation and who may need systemic treatments instead. Catching metastases earlier could also help start the right treatments sooner, potentially improving long-term outcomes.
For patients, this could mean more personalized treatment plans. Those with localized disease could move forward with treatment knowing their cancer has been accurately staged. Meanwhile, those with early metastatic spread could receive targeted therapies right away, reducing the risk of overtreatment or undertreatment. Plus, the longer imaging window of 64Cu-SAR-bisPSMA could make scheduling scans easier, improving access to high-quality imaging.
If the trial is successful, this new imaging agent could become a game-changer for prostate cancer care, helping doctors detect cancer more accurately, make better treatment decisions, and ultimately improve outcomes for patients worldwide.
To learn more about the CLARIFY clinical trial, watch the video we made here.
To learn more, browse our library of prostate cancer-related topics.
Hire PRIMR to create custom video content for your clinical trial or medical product today.
FAQs:
What are the potential limitations or risks associated with using 64Cu-SAR-bisPSMA in clinical practice?
Some studies suggest that copper-based PET tracers may have higher background uptake in the liver, which could make scan interpretation more complex for radiologists. Additionally, because it’s a new radiotracer, hospitals and imaging centers may need special handling procedures and regulatory approvals before it becomes widely available. Smaller medical facilities, in particular, could face hurdles in adopting it due to cost or logistical constraints. Understanding these factors will be essential in determining whether 64Cu-SAR-bisPSMA can complement or even replace current PSMA tracers in everyday clinical practice.
How might 64Cu-SAR-bisPSMA impact treatment decisions beyond surgery and radiation therapy?
More precise imaging could have a ripple effect on many aspects of prostate cancer treatment. If 64Cu-SAR-bisPSMA detects small metastatic tumors earlier than other tracers, patients may start systemic treatments, like hormone therapy, chemotherapy, or targeted radiopharmaceuticals, sooner, rather than opting for aggressive local treatments first. This could lead to a shift in how high-risk or early metastatic prostate cancer is managed. Additionally, repeated scans with this tracer could allow doctors to track a patient’s response to treatment more effectively, helping to adjust therapy plans in real time. In the long run, this could make prostate cancer care even more personalized and adaptable.
How does 64Cu-SAR-bisPSMA compare to emerging non-radioactive imaging techniques for prostate cancer?
PSMA PET imaging is currently one of the most powerful tools for detecting prostate cancer spread, but new technologies are being developed that could complement or even compete with it. AI-enhanced MRI, for instance, is showing promise in detecting prostate tumors with greater accuracy, while liquid biopsies, which look for cancer-related genetic material in the bloodstream, could help identify metastases without the need for radiation. If these approaches continue to evolve, they could provide additional ways to detect and monitor prostate cancer. As these technologies develop, it will be important to understand how 64Cu-SAR-bisPSMA fits into the broader landscape of prostate cancer diagnostics and how it can be used alongside or in comparison with these innovations.
Other Posts
Nuclear Medicine: PSMA Treatment Explained from a Doctor’s Perspective
Nuclear Medicine: PSMA Imaging and Its Impact on Prostate Cancer Care