Node-positive Prostate Cancer and the INNOVATE Trial

By: David Grew MD MPH
"...do I have prostatectomy surgery, or avoid the operating room and go with prostate radiation therapy? "
∗ ∗ ∗
When I meet men at the start of their prostate cancer journey, many are stuck on a very tough decision: do I have prostatectomy surgery, or avoid the operating room and go with prostate radiation therapy? Part of the appeal of surgery is we think of it as one and done: you leave the hospital cancer free and all your treatments are in the rearview mirror.
For many patients that is how the story plays out. But what about those men who didn’t have all the cancer removed at surgery? What if the PSA is still elevated after surgery? And what if at the time of surgery, there was cancer that spread to lymph nodes in the pelvis? This high-risk situation can happen, and if it does, it’s not totally clear to doctors which treatments work best to beat back the cancer.
Thankfully a new clinical trial called the INNOVATE trial (also known as NRG GU008) is looking into this exact issue.
We made a trial video PRIMR you can watch here, and below we have a patient-friendly summary - easy to read in lay terms.
You can read the patient-centric video script below:
If you are a patient with lymph node-positive prostate cancer, and have an elevated PSA after surgery, this clinical trial could be for you. The study explores how incorporating a treatment called apalutamide alongside your standard treatment might offer better outcomes.
This extensive clinical trial is open across 270 cancer centers throughout North America.
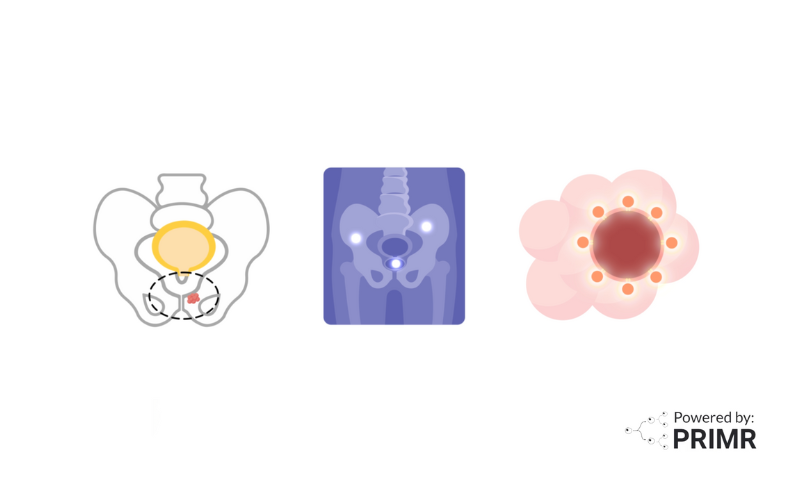
To enroll on this trial, you must have node-positive prostate cancer.
Prostate cancer is node-positive when the cancer leaves the prostate and spreads to lymph nodes in the pelvis.
To be eligible, you must also have an elevated or non-zero PSA after a radical prostatectomy. PSA is a chemical that can be measured in the blood and is often elevated in men with prostate cancer.
Various factors, determined by the physician during the screening process, can affect your eligibility for the trial. These factors may either qualify or disqualify you from participating.
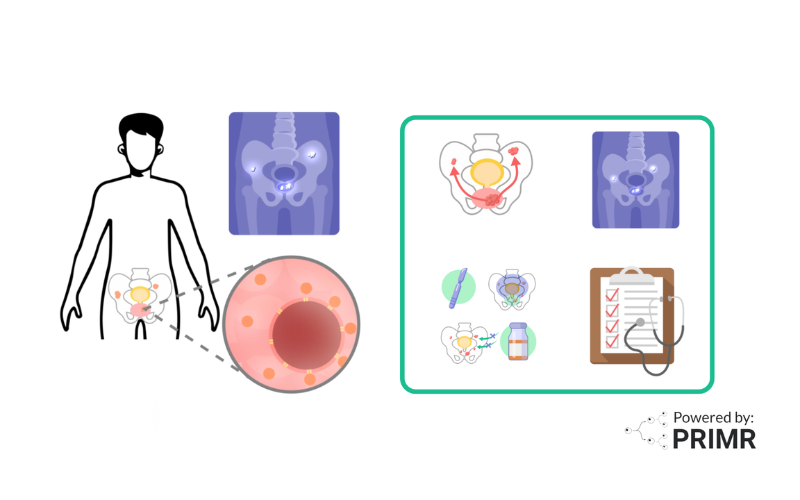
For men who have surgery for prostate cancer, called radical prostatectomy, the PSA should go down to zero after the prostate is removed. Non-zero PSA can be obtained at any time that is more than 30 days after surgery. If the PSA is non-zero or rises after surgery, it is a sign that prostate cancer either was not entirely removed or has grown back. When this happens, the patient is given the standard treatment of radiation and hormone therapy.
Radiation is high-energy X-rays directed to the pelvis. Hormone therapy stops testosterone from fueling the growth of prostate cancer.
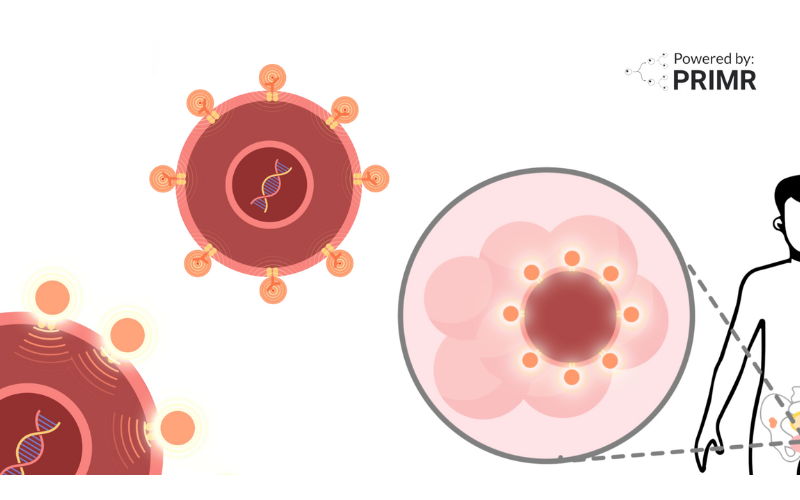
A medication called apalutamide is a pill that is currently FDA-approved for patients with metastatic prostate cancer. Metastatic means it has spread to other parts of the body. Apalutamide can stop the spread and shrink prostate cancer.
A key question in this clinical trial is whether adding apalutamide to your standard treatment will lead to improved cure rates by stopping the spread and shrinking the cancer.
Our success in testing this will only become clear once the trial reaches its conclusion.
In this trial, you will be randomly assigned to receive either standard treatment with radiation therapy and hormone therapy alone, or radiation and hormone therapy with apalutamide.
Neither you nor your doctors get to choose which treatment you receive.
No placebo will be used. Placebo is a pill that contains no active medication. You will receive only active treatment on this trial.
After treatment, doctors will monitor your progress every 6 months for 3 years, then annually thereafter.
If you are interested in helping future patients with prostate cancer receive more effective treatment, the INNOVATE clinical trial may be the right one for you.
The INNOVATE clinical trial is sponsored by the National Cancer Institute. If you have any questions contact NRG Oncology at 267-519-6630 or info@nrgoncology.org.
Thank you for your attention, and be well in your prostate cancer journey.
FAQs:
What are the potential side effects or risks associated with incorporating apalutamide into standard treatment for lymph node-positive prostate cancer patients with elevated PSA after surgery?
This may include common side effects such as fatigue, nausea, diarrhea, or skin rash, as well as more serious but less common adverse effects like cardiovascular events or liver problems. Patients need to discuss these risks with their healthcare providers before considering enrollment in the trial.
How does the INNOVATE trial ensure patient safety and ethical conduct throughout the study, especially considering the use of a new medication like apalutamide?
These are done through rigorous protocols and oversight by institutional review boards (IRBs) at participating cancer centers. These boards review the study design, informed consent process, and patient recruitment to ensure that participants are adequately informed about potential risks and benefits and that their rights and well-being are protected throughout the trial.
What measures are in place to ensure that patients fully understand the nature of the trial, including the random assignment to treatment groups and the absence of a placebo?
The trial employs comprehensive informed consent procedures to ensure that patients understand the nature of the study, including random assignment to treatment groups and the absence of a placebo. Patients receive detailed explanations of the study objectives, procedures, potential risks and benefits, and their rights as participants. Additionally, they have the opportunity to ask questions and discuss any concerns with the research team before deciding whether to enroll.
Other Posts
Nuclear Medicine: PSMA Treatment Explained from a Doctor’s Perspective
Nuclear Medicine: PSMA Imaging and Its Impact on Prostate Cancer Care