Pioneering Precision: The MODERN Trial and Personalized Bladder Cancer Care

By: David Grew MD MPH
“By identifying patients at higher risk of recurrence and tailoring their treatment plans accordingly, the trial addresses a critical need for personalized care in bladder cancer."
∗ ∗ ∗
As a practicing radiation oncologist, I see patients with a new diagnosis of bladder cancer. A frequent challenge lies in balancing aggressive treatment to prevent recurrence with avoiding unnecessary therapies that can negatively affect quality of life.
Consider a patient who has undergone surgery to remove their bladder after a diagnosis of muscle-invasive bladder cancer. Post-surgery, they face an uncertain path—should we pursue additional treatments to prevent recurrence, or is surveillance enough? This dilemma highlights the importance of precision medicine in cancer care.
The MODERN trial seeks to address this by using a cutting-edge approach: circulating tumor DNA (ctDNA) testing. The findings of this trial could potentially redefine post-surgical care for bladder cancer patients, offering hope for more targeted and effective treatments.
Understanding the MODERN Trial
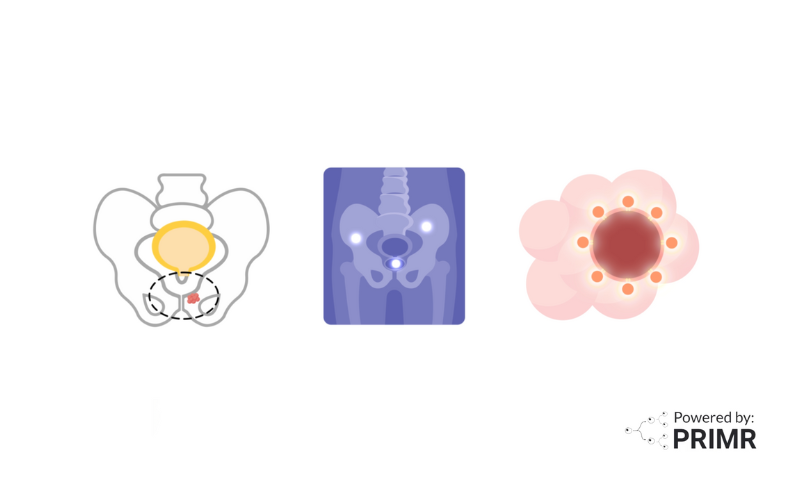
The MODERN trial investigates advanced treatment strategies for patients with muscle-invasive bladder cancer who have undergone surgery. Bladder cancer recurrence is a significant concern, often going undetected until it grows large enough to be visible on scans.
The study uses an innovative blood test called circulating tumor DNA (ctDNA) testing to identify patients at risk for recurrence and tailor their treatment accordingly. By analyzing ctDNA levels, the MODERN trial divides participants into two groups:
- ctDNA-Positive Cohort – Cancer DNA fragments detected in the bloodstream after surgery, signaling a higher risk of recurrence.
- ctDNA-Negative Cohort – No cancer DNA fragments detected in the bloodstream, indicating a lower risk of recurrence.
The trial explores whether ctDNA testing can guide the use of immunotherapy to improve outcomes for patients while minimizing unnecessary treatments.
Standard Treatment and Its Limitations
The standard approach for treating bladder cancer includes surgery to remove the bladder (cystectomy), often followed by chemotherapy or immunotherapy for advanced stages. Patients are then monitored using regular imaging tests like CT scans to detect recurrence.
However, this method has limitations. Small amounts of cancer cells that could lead to recurrence may not show up on scans until much later. The MODERN trial addresses this gap by utilizing ctDNA testing, which can detect microscopic traces of cancer earlier than imaging techniques. This innovation opens the door to more personalized treatment strategies, balancing effective care with the avoidance of unnecessary side effects.
How ctDNA Testing Works
Circulating tumor DNA (ctDNA) testing involves analyzing a blood sample for small fragments of DNA shed by cancer cells into the bloodstream. This technology detects microscopic traces of cancer that are often undetectable with imaging methods like CT scans.
Studies have shown ctDNA testing to be highly sensitive for identifying residual disease and predicting recurrence. However, its reliability depends on factors like the tumor's mutation profile and the volume of ctDNA present.
It complements, rather than replaces, traditional imaging by identifying risks earlier.
The ctDNA-Positive Treatment Arm
For patients in the ctDNA-positive cohort, cancer DNA fragments are detectable in their bloodstream after surgery. This finding suggests that cancer cells may still be present in the body, increasing the risk of recurrence.
Participants in this group are randomized into two treatment arms:
- Standard Treatment Groupsome text
- Immunotherapy with nivolumab for up to 12 months.
- Nivolumab is administered through an IV every 28 days, helping the immune system identify and attack cancer cells.
- Enhanced Treatment Groupsome text
- Combination immunotherapy with nivolumab and relatlimab for up to 12 months.
- Relatlimab works by targeting additional immune signals, potentially enhancing the body’s ability to fight cancer.
This part of the trial aims to determine if the combination of nivolumab and relatlimab offers greater protection against recurrence than nivolumab alone. Regular follow-ups, including blood tests and scans, help monitor the effectiveness of each treatment approach.
By focusing on this high-risk group, the MODERN trial seeks to establish whether intensified treatment can lead to better long-term outcomes for patients with ctDNA-positive results.
The ctDNA-Negative Treatment Arm
Patients in the ctDNA-negative cohort do not have detectable cancer DNA fragments in their bloodstream, indicating a lower risk of recurrence. For this group, the trial examines whether additional treatment is necessary or if active surveillance is sufficient.
Participants in this group are randomized into two treatment arms:
- Immunotherapy Groupsome text
- Treatment with nivolumab for up to 12 months.
- Surveillance Groupsome text
- No immediate treatment after surgery. Patients undergo close monitoring with regular blood tests and scans to check for signs of recurrence.
- If ctDNA becomes detectable during follow-up, these patients may begin immunotherapy with nivolumab.
This part of the trial aims to ensure that patients with a lower risk of recurrence can avoid unnecessary treatments and their associated side effects while still receiving aggressive care if their cancer shows signs of returning.
Why the MODERN Trial Matters
The MODERN trial represents a significant step forward in using ctDNA technology to guide bladder cancer treatment. By identifying patients at higher risk of recurrence and tailoring their treatment plans accordingly, the trial addresses a critical need for personalized care.
For patients, the implications are profound. Early detection through ctDNA testing means a better chance of preventing recurrence, while unnecessary treatments and their side effects can be avoided for those at lower risk. This approach could set a new standard for post-surgical care in bladder cancer, offering patients hope for improved outcomes and a higher quality of life.
If successful, the MODERN trial has the potential to revolutionize how we treat bladder cancer, paving the way for more precise and effective therapies.
To learn more about the MODERN clinical trial, watch the 3-part series we made here.
To learn more about circulating tumor DNA, watch the short video we made here.
Hire PRIMR to create custom video content for your clinical trial or medical product today.
FAQs:
What are the most common side effects of nivolumab and relatlimab, and how are they managed?
Nivolumab and relatlimab are treatments that help the immune system attack cancer, but they can sometimes cause the immune system to become overactive. Common side effects include fatigue, rash, diarrhea, and inflammation of organs such as the lungs (pneumonitis), liver (hepatitis), or thyroid (thyroiditis). Management typically involves corticosteroids or other immunosuppressive medications to reduce inflammation. Patients in trials like MODERN are closely monitored for these effects to ensure timely intervention.
How does the MODERN trial address the challenge of overtreatment in cancer care?
The MODERN trial helps reduce overtreatment by dividing patients into groups based on their risk of cancer coming back, as determined by ctDNA testing. Patients in the low-risk group (ctDNA-negative) may avoid unnecessary treatments unless their risk changes over time. This approach helps patients avoid the side effects and stress of treatments they don’t need, while still providing close monitoring and care if their cancer shows signs of returning. For those at higher risk, treatment is more intensive, personalizing treatment while aiming to achieve better outcomes.
What are the potential limitations of the MODERN trial's approach, and how might they affect its findings?
The MODERN trial has some challenges. For example, ctDNA testing may not always detect cancer if the amount of DNA in the blood is very small or if the test isn’t sensitive enough. There’s also a chance of false results—some tests might miss cancer that’s present (false negative), or detect cancer when it’s not really there (false positive). Additionally, the trial’s results might not apply to all bladder cancer patients, especially those with unusual types of tumors or who can’t receive immunotherapy. These challenges highlight the importance of combining ctDNA testing with other tools and continuing research to improve treatment for all patients.
Other Posts
Nuclear Medicine: PSMA Treatment Explained from a Doctor’s Perspective
Nuclear Medicine: PSMA Imaging and Its Impact on Prostate Cancer Care