Radiopharma Series Part 3: Mental Framework for Understanding Radiopharmaceuticals

By: David Grew MD MPH
“One of the most effective ways to wrap your head around radiopharmaceuticals is to categorize them based on their primary applications and mechanisms of action.”
∗ ∗ ∗
When I was in medical school, and right on through residency, I realized the volume of information I was responsible for was too overwhelming to digest at once. So I had to develop a mental model to categorize things in my brain. That way if (when) I got lost, I had a simple framework to lean on to keep disparate threads of information straight. It worked reasonably well for exams, and I still use this strategy to this day when I meet with my own cancer patients.
In this, the 3rd blog in our series on radiopharmaceuticals, I’ll lay out the framework that works for me to organize the different categories and use cases for radiopharma mentally.
–
One of the most effective ways to wrap your head around radiopharmaceuticals is to categorize them based on their primary applications and mechanisms of action. This approach not only simplifies the complexity of this drug class but also enhances knowledge retention and practical application.
The first category encompasses Diagnostic Radiopharmaceuticals, which are further divided into two subcategories:
Imaging Agents: These radiopharmaceuticals are designed for diagnostic imaging purposes, allowing visualization of specific organs, tissues, or biological processes within the body. Examples include Technetium-99m (⁹⁹ᵐTc) labeled agents for imaging various organs like the brain, heart, lungs, and bones, as well as Fluorine-18 (18F) labeled radiopharmaceuticals for positron emission tomography (PET) imaging, such as 18F-FDG for cancer detection and staging.
Functional Imaging Agents: These radiopharmaceuticals are used to assess the physiological function or metabolic activity of specific organs or tissues. For instance, Iodine-123 (123I) or Technetium-99m (⁹⁹ᵐTc) labeled agents can evaluate thyroid function, while radiolabeled neurotransmitter analogs can help diagnose neurological disorders like Parkinson's disease.
The second major category is Therapeutic Radiopharmaceuticals, which can be further divided into two subcategories:
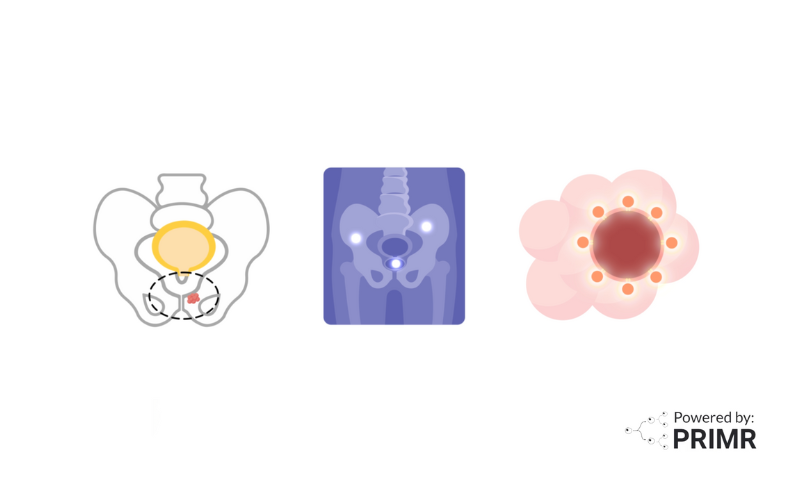
Targeted Radionuclide Therapy: These radiopharmaceuticals are designed to deliver therapeutic doses of radiation directly to specific target cells or tissues, such as cancer cells. Examples include Lutetium-177 (177Lu) or Yttrium-90 (90Y) labeled peptides for neuroendocrine tumor treatment, like 177Lu-DOTATATE, and Radium-223 (223Ra) for treating bone metastases in prostate cancer.
Radioimmunotherapy: These radiopharmaceuticals combine the highly specific targeting ability of monoclonal antibodies with the therapeutic effects of radiation. Ibritumomab tiuxetan (90Y-Zevalin) and Tositumomab (131I-Bexxar) are examples used in the treatment of non-Hodgkin's lymphoma.
The third and newest category of radiopharma is Theranostic Pairs, which combines diagnostic imaging and therapeutic intervention by utilizing matched pairs of radiopharmaceutical agents that target the same biological process or molecular target.
Theranostic Pairs are diagnostic and therapeutic radiopharmaceuticals that access the same cellular or biological pathway with a matched pair of radionuclides. Some examples include:
- 68Ga for PET imaging and 177Lu for radionuclide therapy, both coupled to ligands targeting somatostatin receptors (SSTR) or prostate-specific membrane antigen (PSMA) in neuroendocrine tumors and prostate cancer, respectively.
- 123I/124I for imaging and 131I for therapy, targeting sodium/iodide symporters in thyroid cancer.
- 111In for imaging and 90Y for radioimmunotherapy, both conjugated to anti-CD20 monoclonal antibodies in non-Hodgkin's lymphoma.
The power of theranostic pairs lies in their ability to precisely stratify patients, plan personalized treatments, and monitor therapeutic responses, all while leveraging the same molecular target. This integrated approach not only enhances treatment efficacy, but also optimizes resource allocation and improves overall patient outcomes.
Whenever I find myself overwhelmed by the complexities of radiopharmaceuticals, I return to this mental framework. It serves as a reliable anchor, helping me navigate the intricate details while maintaining a clear overview of the field.
Whether I'm preparing for a patient consultation, staying up-to-date with the latest developments, or helping our industry partners build educational resources for patients and providers, this simple yet effective categorization system has proven invaluable in my journey as a cancer specialist.
Radioiodine Therapy for Thyroid Cancer
Radioiodine therapy is a prime example of how radiopharmaceuticals can harness the body's natural processes to deliver targeted radiation directly to cancer cells. In this case, the therapy takes advantage of the thyroid gland's unique ability to absorb iodine. By administering radioactive iodine (131I), the treatment precisely targets and destroys thyroid cancer cells while minimizing harm to surrounding healthy tissues.
The success of radioiodine therapy lies in its remarkable precision and efficacy. It has proven invaluable in managing both localized and metastatic thyroid cancer, offering patients a powerful tool in their fight against this disease. Moreover, the treatment is generally well-tolerated, making it an attractive option for many individuals.
Targeted Radionuclide Therapies: Precision Strikes Against Cancer
Building upon the success of radioiodine therapy, the field of radiopharmaceuticals has witnessed remarkable advancements in recent years. Targeted radionuclide therapies represent a new frontier in cancer treatment, harnessing the power of precision medicine to deliver targeted radiation directly to tumor sites.
Thanks to groundbreaking research in molecular biology and cancer science, we now have a deeper understanding of the specific molecular targets and pathways involved in tumor growth and progression. This knowledge has paved the way for the development of radiopharmaceuticals that can selectively bind to cancer cells, delivering therapeutic doses of radiation with pinpoint accuracy.
The applications of targeted radionuclide therapies are diverse and promising. One notable example is peptide receptor radionuclide therapy (PRRT), which has shown remarkable efficacy in treating neuroendocrine tumors. Additionally, alpha particle therapy has emerged as a promising approach for prostate cancer, while radiolabeled antibodies have demonstrated success in treating lymphoma and leukemia.
These targeted therapies represent a paradigm shift in cancer care, offering patients a more personalized and effective treatment option. By precisely targeting cancer cells while minimizing harm to healthy tissues, targeted radionuclide therapies have the potential to improve outcomes and enhance quality of life for countless individuals battling this formidable disease.
Theranostics: Unlocking the Power of Personalized Cancer Care
A revolutionary concept known as theranostics is now emerging, representing a true paradigm shift in cancer care. This approach combines diagnostic imaging with therapeutic intervention, utilizing the same or similar radiopharmaceutical agents for both purposes.
At the heart of theranostics lies the concept of precision medicine. By leveraging theranostic pairs, such as 68Ga-DOTATATE for imaging and 177Lu-DOTATATE for therapy in neuroendocrine tumors, healthcare professionals can precisely stratify patients, plan treatments, and monitor responses with unprecedented accuracy.
The beauty of theranostics lies in its integrated and personalized approach. Not only does it enhance treatment efficacy by tailoring interventions to each individual patient's molecular tumor signature, but it also optimizes resource allocation and improves overall patient outcomes. By combining diagnostic and therapeutic capabilities into a single platform, theranostics streamlines the entire process, ensuring that patients receive the right treatment at the right time.
Moreover, theranostics opens up new avenues for research and development, enabling scientists and clinicians to explore novel radiopharmaceutical agents and refine existing ones. This continuous innovation holds the promise of even more effective and targeted cancer treatments in the future, further advancing the field of personalized medicine.
The Future of Radiopharmaceuticals: Emerging Technologies and Collaborations
The field of radiopharmaceuticals is rapidly evolving, driven by ongoing research efforts focused on expanding the repertoire of available treatments, refining targeting strategies, and enhancing treatment delivery and monitoring techniques.
Advances in nanotechnology, radiochemistry, and radiobiology hold significant promise for the development of next-generation radiopharmaceuticals. These cutting-edge technologies have the potential to improve pharmacokinetics, enhance tumor penetration, and reduce off-target toxicity, ultimately leading to more effective and safer cancer treatments.
Collaborative initiatives across academia, industry, and regulatory agencies are playing a crucial role in accelerating the translation of novel radiopharmaceuticals from bench to bedside. By fostering interdisciplinary collaborations and streamlining the development and approval processes, these initiatives are driving innovation and improving outcomes for patients with cancer.
Looking ahead, the future of radiopharmaceuticals is bright, with numerous exciting developments on the horizon. From targeted alpha therapies to novel radionuclide conjugates and advanced imaging techniques, the field is poised to make significant strides in the fight against cancer, offering new hope and improved quality of life for patients worldwide.
Other Posts
Nuclear Medicine: PSMA Treatment Explained from a Doctor’s Perspective
Nuclear Medicine: PSMA Imaging and Its Impact on Prostate Cancer Care