Tailoring Prostate Cancer Treatment for Better Outcomes with the INDICATE Trial

By: David Grew MD MPH
“A PET scan offers a more detailed look at cancer spread compared to traditional imaging methods like CT or MRI."
∗ ∗ ∗
As a radiation oncologist, I have witnessed the challenges patients face when prostate cancer returns after surgery. Imagine a patient who has undergone surgery and standard treatment, only to see their prostate-specific antigen (PSA) levels rise again—a harbinger that cancer may be back. Such scenarios underscore the urgency for more personalized and effective treatment strategies.
The INDICATE trial explores new ways to optimize treatment for patients in this situation, particularly by tailoring approaches based on PET scan findings. This trial has the potential to transform how we manage recurrent prostate cancer and offer hope to patients and their families. INDICATE is being conducted by ECOG-ACRIN Cancer Research Group and the lead investigator is Dr. Neha Vapiwala of the University of Pennsylvania.
Let’s dive into the key aspects of the trial, its goals, and how it’s structured to improve patient outcomes.
Understanding the INDICATE Trial
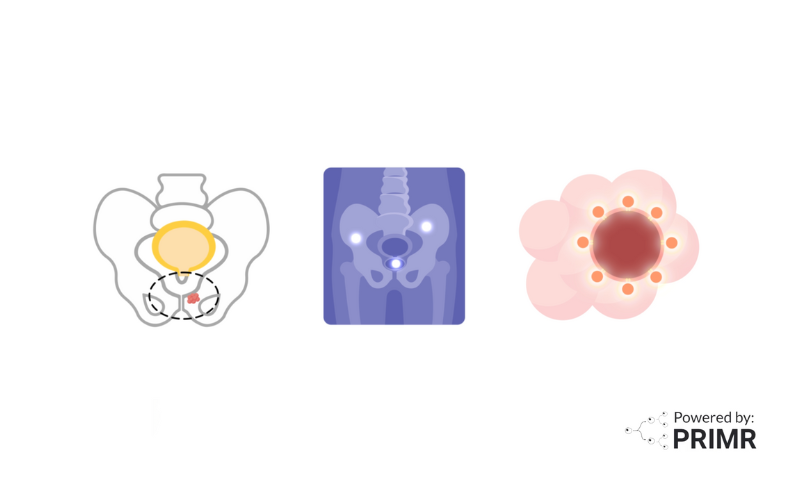
The INDICATE trial investigates advanced treatment options for patients whose prostate cancer has returned after surgery. Prostate cancer recurrence is often identified through a rise in PSA levels, and PET scans are used to detect whether the cancer has spread beyond the pelvis.
The study divides participants into two groups based on PET scan results:
- PET-Negative Cohort – No metastases outside the pelvis detectable on PET scans.
- PET-Positive Cohort – Metastases outside the pelvis detectable only on PET scans.
The trial examines whether adjusting treatment protocols for these two groups can improve outcomes, such as delaying cancer progression or extending survival.
The Standard Approach and Its Challenges
Standard treatment for recurrent prostate cancer includes radiation therapy (RT) to the pelvic area and androgen deprivation therapy (ADT). While this approach can be effective, some patients still experience recurrence, highlighting the need for improved strategies.
A PET scan offers a more detailed look at cancer spread compared to traditional imaging methods like CT or MRI. However, it also raises questions about how to treat cancer that is detectable only on PET scans. The INDICATE trial seeks to answer this critical question by testing innovative approaches to treatment tailored to PET scan findings.
Exploring the PET-Negative Cohort
For patients in the PET-negative cohort, no metastases outside the pelvis are visible on PET scans. These patients are randomized into two groups:
1. Standard Treatment Group
a. External beam RT targeting the pelvic lymph nodes and prostate bed
b. Six months of ADT to block hormone-related cancer growth
2. Enhanced Treatment Group
a. Standard treatment (RT + ADT)
Addition of apalutamide, an FDA-approved medication that blocks other hormone-related signals fueling cancer growth.
The goal of this part of the trial is to determine whether adding apalutamide can further improve outcomes for patients without visible metastases. The study closely monitors patients for side effects and treatment response, with regular follow-ups over several years.
By addressing the question of whether apalutamide offers additional benefits, this part of the trial could set a new standard for managing prostate cancer that appears localized on PET scans.
Addressing the PET-Positive Cohort
Patients in the PET-positive cohort present a unique challenge, as their cancer has spread beyond the pelvis but is detectable only on PET scans. This group is also randomized into two treatment arms:
1. Standard Treatment with Apalutamide
a. RT targeting the pelvic lymph nodes and prostate bed
b. Six months of ADT
c. Addition of apalutamide for six months
2. Enhanced Treatment with MDT
a. Standard treatment with RT, ADT, and apalutamide
b. Addition of metastasis-directed radiation therapy (MDT) to target visible sites of metastatic cancer
MDT has shown promise in delaying cancer progression and extending survival for some patients with metastatic cancer. This part of the INDICATE trial aims to evaluate whether combining MDT with standard treatment can lead to better outcomes for patients with PET-detectable metastases.
Why the INDICATE Trial Matters
The primary goal of the INDICATE trial is to improve treatment outcomes for men with recurrent prostate cancer by using PET scan technology to guide therapy. By tailoring treatments based on precise imaging data, this trial has the potential to revolutionize how recurrent prostate cancer is managed—whether the disease is localized or has spread.
For patients facing recurrent prostate cancer, receiving personalized treatment can be a game-changer. The INDICATE trial addresses this need by exploring advanced imaging and targeted therapies, with the hope of reducing recurrences and improving survival. If successful, this trial could significantly enhance the standard of care for prostate cancer patients, offering better outcomes and improved quality of life.
To learn more about INDICATE, watch the 3-part series we made here.
Hire PRIMR to create custom video content for your clinical trial or medical product today.
FAQs:
What is the difference between a PET scan and traditional imaging methods like CT or MRI for prostate cancer detection?
A PET (Positron Emission Tomography) scan uses a radioactive tracer, such as PSMA (prostate-specific membrane antigen), to detect cancer cells that absorb the tracer. It provides more detailed imaging, especially for small or early-stage metastases, compared to CT or MRI. While CT and MRI are excellent for visualizing the size and location of tumors, PET scans can detect biochemical activity, making them particularly useful for recurrent cancer where early metastasis may be difficult to see on other imaging methods.
What are the potential side effects of adding apalutamide to the treatment regimen?
Apalutamide, an androgen receptor inhibitor, can cause side effects such as fatigue, skin rash, hot flashes, joint pain, decreased appetite, weight loss, and, in rare cases, cardiovascular events like hypertension. Patients in the INDICATE trial are closely monitored to balance the benefits of the medication with its potential risks.
How does metastasis-directed therapy (MDT) differ from standard radiation therapy in prostate cancer treatment?
MDT focuses radiation specifically on sites of metastatic cancer identified through imaging, rather than broadly targeting the prostate bed and pelvic lymph nodes. This targeted approach aims to eradicate visible metastatic lesions, potentially slowing disease progression and prolonging survival, especially in patients with oligometastatic disease (a limited number of metastases).
Other Posts
Nuclear Medicine: PSMA Treatment Explained from a Doctor’s Perspective
Nuclear Medicine: PSMA Imaging and Its Impact on Prostate Cancer Care